Introduction
The Earth’s crust contains a remarkable diversity of chemical minerals that form the foundation of modern civilization. Among these, lithium, platinum, silver, gold, copper, and manganese stand out as critical elements that power our technologies, economies, and industrial processes. This comprehensive article explores these six essential chemical minerals, their properties, occurrences, extraction methods, and the vast array of mineral compounds derived from them.
1. Lithium: The Lightest Metal
Overview and Properties
Lithium (Li, atomic number 3) is the lightest metal and the least dense solid element at room temperature. Its exceptional electrochemical properties have made it indispensable in modern energy storage technology. Lithium’s atomic structure allows it to readily give up its single valence electron, making it highly reactive and ideal for battery applications.
Primary Lithium Minerals
Lithium occurs in nature primarily through the following minerals:
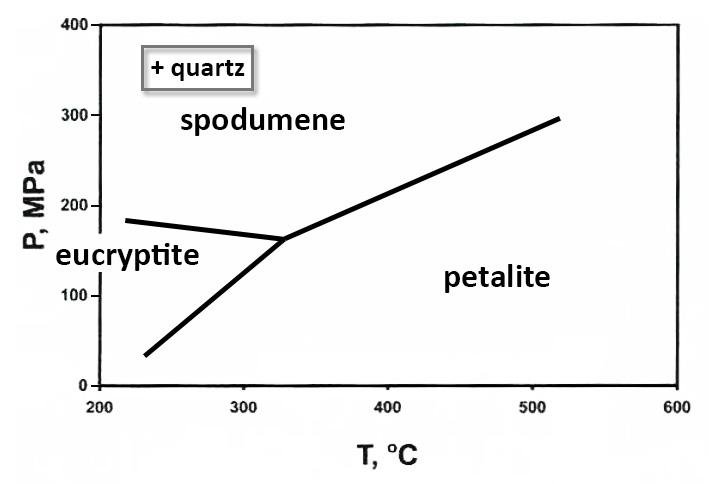
Spodumene (LiAlSi₂O₆): The most important lithium ore mineral, spodumene is a pyroxene mineral that typically contains 6-7% lithium oxide. It forms in lithium-rich pegmatites and can appear in various colors including pink (kunzite), green (hiddenite), and yellow varieties.
Lepidolite (K(Li,Al)₃(Si,Al)₄O₁₀(F,OH)₂): A lilac-gray or pink lithium-rich mica containing 3-4% lithium. Lepidolite is commonly found in granite pegmatites and represents an important secondary source of lithium.
Petalite (LiAlSi₄O₁₀): A white to gray lithium aluminum silicate containing approximately 4.5% lithium oxide. Petalite is valued for its use in glass and ceramics production due to its low thermal expansion properties.
Amblygonite-Montebrasite ((Li,Na)AlPO₄(F,OH)): A lithium aluminum phosphate mineral series containing 8-10% lithium oxide, often found in lithium-bearing pegmatites.
Eucryptite (LiAlSiO₄): A rare lithium aluminum silicate with about 11.8% lithium oxide content, typically found as an alteration product of spodumene.
Lithium Brine Sources
Beyond hard rock minerals, lithium is extracted from brine deposits, which contain dissolved lithium salts:
- Lithium chloride (LiCl) in salt lake brines
- Lithium carbonate (Li₂CO₃) in evaporite deposits
- Lithium sulfate (Li₂SO₄) in certain brine environments
Secondary Lithium Compounds and Minerals
From primary lithium minerals, numerous compounds are produced:
- Lithium hydroxide (LiOH): Critical for battery cathode production
- Lithium bromide (LiBr): Used in air conditioning and dehumidification
- Lithium fluoride (LiF): Applications in ceramics and specialized optics
- Lithium nitrate (LiNO₃): Used in ceramics and pyrotechnics
- Lithium oxide (Li₂O): Essential in glass and ceramics
- Lithium phosphate (Li₃PO₄): Battery cathode material
- Lithium cobalt oxide (LiCoO₂): Common battery cathode material
- Lithium iron phosphate (LiFePO₄): Safer battery cathode alternative
- Lithium nickel manganese cobalt oxide (NMC): Advanced battery material
Total lithium-derived minerals and compounds: Approximately 15-20 major varieties
2. Platinum: The Noble Metal
Overview and Properties
Platinum (Pt, atomic number 78) is one of the rarest elements in Earth’s crust, occurring at concentrations of only about 0.005 parts per million. This silvery-white metal belongs to the platinum group metals (PGMs) and exhibits exceptional resistance to corrosion and high temperatures. Its catalytic properties and rarity make it one of the most valuable industrial metals.
Primary Platinum Minerals
Platinum rarely occurs as a pure native element and is more commonly found in various mineral forms:
Sperrylite (PtAs₂): Platinum arsenide, the most common platinum-bearing mineral, containing about 56.5% platinum. It forms as cubic crystals in magmatic deposits.
Cooperite (PtS): Platinum sulfide containing approximately 81% platinum, commonly found in the Bushveld Complex of South Africa.
Braggite ((Pt,Pd,Ni)S): A platinum-palladium-nickel sulfide that forms a solid solution series with cooperite.
Native Platinum (Pt with Fe, Ir, Rh, Pd): Pure metallic platinum naturally alloyed with iron and other platinum group metals, occurring as nuggets or grains.
Isoferroplatinum (Pt₃Fe): An iron-platinum alloy mineral containing 76-81% platinum.
Tetraferroplatinum (PtFe): An equimolar platinum-iron mineral.
Stibiopalladinite (Pd₅Sb₂): While primarily a palladium mineral, often associated with platinum deposits.
Platinum Group Minerals (PGMs)
Platinum is rarely found alone and occurs with other platinum group elements:
Palladium Minerals:
- Palladium (Pd) native metal
- Potarite (PdHg)
- Stibiopalladinite (Pd₅Sb₂)
Rhodium Minerals:
- Rhodium (Rh) as native metal or in platinum alloys
- Rhodplumsite (Rh₃Pb₂)
Iridium Minerals:
- Iridium (Ir) native metal
- Irarsite (IrAsS)
- Osmiridium ((Os,Ir) alloy)
Osmium Minerals:
- Osmium (Os) native metal
- Osmiridium (Os,Ir alloy)
Ruthenium Minerals:
- Ruthenium (Ru) native metal
- Laurite (RuS₂)
Platinum Compounds
Industrial processes create numerous platinum compounds:
- Platinum oxide (PtO and PtO₂): Catalytic applications
- Platinum chloride (PtCl₂ and PtCl₄): Chemical synthesis
- Hexachloroplatinic acid (H₂PtCl₆): Platinum plating and catalyst preparation
- Platinum sulfide (PtS): Naturally occurring mineral form
- Platinum complexes: Various coordination compounds used in medicine (cisplatin) and catalysis
- Platinum-rhodium alloys: High-temperature applications
- Platinum-iridium alloys: Measurement standards and crucibles
Total platinum-derived minerals and compounds: Approximately 25-30 major varieties
3. Silver: The White Metal
Overview and Properties
Silver (Ag, atomic number 47) has been prized by human civilizations for over 5,000 years. It possesses the highest electrical and thermal conductivity of all metals and exhibits exceptional reflectivity. Silver’s antimicrobial properties, combined with its industrial applications, make it both a precious and industrial metal.
Primary Silver Minerals
Silver occurs in over 200 different mineral species:
Native Silver (Ag): Pure metallic silver found in wire, plate, and dendritic forms, often containing minor copper or gold inclusions.
Argentite/Acanthite (Ag₂S): Silver sulfide, one of the most important silver ore minerals containing 87% silver. Argentite is the high-temperature form, while acanthite is stable at lower temperatures.
Proustite (Ag₃AsS₃): Light ruby silver, a silver arsenic sulfosalt containing 65.4% silver, known for its deep red color.
Pyrargyrite (Ag₃SbS₃): Dark ruby silver, a silver antimony sulfosalt with 59.9% silver content.
Chlorargyrite (AgCl): Silver chloride, also called horn silver, containing 75.3% silver, commonly found in oxidized zones of silver deposits.
Bromyrite (AgBr): Silver bromide, containing 57.4% silver, rare but similar to chlorargyrite in occurrence.
Iodyrite (AgI): Silver iodide, extremely rare, containing 45.9% silver.
Stephanite (Ag₅SbS₄): Silver antimony sulfosalt with 68.5% silver.
Polybasite ((Ag,Cu)₁₆Sb₂S₁₁): Complex silver antimony sulfosalt containing 60-75% silver.
Pearceite ((Ag,Cu)₁₆As₂S₁₁): Silver arsenic sulfosalt similar to polybasite.
Additional Silver-Bearing Minerals
Tetrahedrite-Tennantite Series ((Cu,Fe,Ag,Zn)₁₂Sb₄S₁₃ to (Cu,Fe,Ag,Zn)₁₂As₄S₁₃): Copper antimony/arsenic sulfosalts that can contain significant silver.
Cerargyrite (AgCl): Another name for chlorargyrite.
Stromeyerite (AgCuS): Copper silver sulfide with about 53% silver.
Dyscrasite (Ag₃Sb): Silver antimonide containing 78.1% silver.
Naumannite (Ag₂Se): Silver selenide, rare but important in some deposits.
Aguilarite (Ag₄SeS): Silver selenium sulfide.
Hessite (Ag₂Te): Silver telluride containing 63% silver.
Petzite (Ag₃AuTe₂): Silver gold telluride.
Jalpaite (Ag₃CuS₂): Silver copper sulfide.
Silver Compounds
Modern industry produces numerous silver compounds:
- Silver nitrate (AgNO₃): Photography, medicine, and chemical synthesis
- Silver oxide (Ag₂O): Batteries and catalysis
- Silver halides (AgCl, AgBr, AgI): Traditional photography
- Silver sulfate (Ag₂SO₄): Analytical chemistry
- Silver acetate (AgC₂H₃O₂): Organic synthesis
- Silver carbonate (Ag₂CO₃): Chemical reactions
- Silver chromate (Ag₂CrO₄): Analytical indicator
- Silver fulminate (AgCNO): Historical explosive
- Silver nanoparticles: Antimicrobial applications
- Colloidal silver: Antimicrobial suspensions
Total silver-derived minerals and compounds: Approximately 200+ natural minerals and 30+ synthetic compounds
4. Gold: The King of Metals
Overview and Properties
Gold (Au, atomic number 79) has captivated humanity for millennia. Its unique combination of beauty, malleability, corrosion resistance, and rarity has made it the ultimate store of value across cultures. Gold’s chemical inertness means it occurs primarily in its native metallic form, though several important gold-bearing minerals exist.
Primary Gold Minerals
Native Gold (Au): Pure gold (typically 80-95% pure when naturally occurring) found in veins, placers, and disseminated deposits. Natural gold almost always contains some silver, forming a continuous solid solution called electrum.
Electrum (Au,Ag): Natural gold-silver alloy containing 20-50% silver. The color transitions from golden to pale yellow as silver content increases.
Calaverite (AuTe₂): Gold telluride containing 39.6% gold, one of the few chemical compounds of gold found in nature.
Sylvanite ((Au,Ag)₂Te₄): Gold silver telluride with variable gold content (24-28%), typically appearing with a silver-white color.
Krennerite (Au₃AgTe₈): Gold silver telluride containing about 36% gold.
Nagyagite (Pb₅Au(Te,Sb)₄S₅₋₈): Complex gold lead telluride sulfide.
Petzite (Ag₃AuTe₂): Silver-rich gold telluride containing about 25% gold.
Montbrayite ((Au,Sb)₂Te₃): Gold antimony telluride.
Aurostibite (AuSb₂): Gold antimonide, rare but distinctive.
Maldonite (Au₂Bi): Gold bismuthide, very rare.
Fischesserite (Ag₃AuSe₂): Gold silver selenide.
Gold in Other Minerals
Gold commonly occurs as microscopic inclusions in:
- Pyrite (FeS₂): “Fool’s gold” often contains invisible gold particles
- Arsenopyrite (FeAsS): Important host for refractory gold
- Chalcopyrite (CuFeS₂): Copper iron sulfide with gold inclusions
- Stibnite (Sb₂S₃): Antimony sulfide associated with gold
- Realgar (As₄S₄): Arsenic sulfide in some gold deposits
- Orpiment (As₂S₃): Another arsenic mineral associated with gold
Gold Compounds
Unlike most metals, gold forms relatively few stable compounds:
- Gold(I) chloride (AuCl): Unstable compound used in synthesis
- Gold(III) chloride (AuCl₃): Used in gold plating and medicine
- Chloroauric acid (HAuCl₄): Gold plating and nanoparticle synthesis
- Gold(I) cyanide (AuCN): Formed during gold extraction
- Gold(III) oxide (Au₂O₃): Purple compound used in coloring glass
- Gold hydroxide (Au(OH)₃): Intermediate in gold chemistry
- Gold nanoparticles: Various sizes for medical and technological applications
- Colloidal gold: Ruby-colored gold suspensions
- Gold amalgams (Au-Hg): Historical gold extraction method
- Organogold compounds: Various gold complexes used in medicine and catalysis
Total gold-derived minerals and compounds: Approximately 30-40 natural minerals and 20+ synthetic compounds
5. Copper: The Red Metal
Overview and Properties
Copper (Cu, atomic number 29) was among the first metals used by humans, marking the transition from the Stone Age to the Bronze Age. Its excellent electrical conductivity, malleability, and relative abundance make it essential for modern civilization. Copper occurs in numerous oxidation states and forms an extensive array of minerals.
Primary Copper Sulfide Minerals
Chalcopyrite (CuFeS₂): The most abundant copper ore mineral, containing 34.6% copper. Its brassy yellow color and metallic luster make it distinctive.
Bornite (Cu₅FeS₄): Also called “peacock ore” due to its iridescent tarnish, containing 63.3% copper.
Chalcocite (Cu₂S): One of the richest copper ores with 79.8% copper content, typically forming in enriched zones.
Covellite (CuS): Indigo-blue copper sulfide containing 66.5% copper, commonly forming as an alteration product.
Digenite (Cu₉S₅): High-temperature copper sulfide containing about 78% copper.
Djurleite (Cu₁.₉₇S): Copper sulfide similar to chalcocite.
Enargite (Cu₃AsS₄): Copper arsenic sulfosalt with 48.4% copper.
Tetrahedrite ((Cu,Fe)₁₂Sb₄S₁₃): Gray copper ore containing 30-45% copper plus silver.
Tennantite ((Cu,Fe)₁₂As₄S₁₃): Arsenic analog of tetrahedrite.
Copper Oxide and Carbonate Minerals
Cuprite (Cu₂O): Ruby copper ore containing 88.8% copper, one of the richest copper ores.
Tenorite (CuO): Black copper oxide with 79.9% copper content.
Malachite (Cu₂CO₃(OH)₂): Brilliant green copper carbonate hydroxide containing 57.5% copper, highly prized as a gemstone.
Azurite (Cu₃(CO₃)₂(OH)₂): Azure-blue copper carbonate hydroxide with 55.3% copper.
Chrysocolla ((Cu,Al)₂H₂Si₂O₅(OH)₄·nH₂O): Blue-green copper silicate, variable composition.
Additional Copper Minerals
Native Copper (Cu): Pure metallic copper found in massive, dendritic, or crystalline forms.
Atacamite (Cu₂Cl(OH)₃): Green copper chloride hydroxide.
Brochantite (Cu₄SO₄(OH)₆): Green copper sulfate hydroxide.
Antlerite (Cu₃SO₄(OH)₄): Another green copper sulfate hydroxide.
Chalcanthite (CuSO₄·5H₂O): Blue copper sulfate pentahydrate, water-soluble.
Dioptase (CuSiO₃·H₂O): Emerald-green copper silicate, gem-quality.
Turquoise (CuAl₆(PO₄)₄(OH)₈·4H₂O): Blue to green copper aluminum phosphate, popular gemstone.
Olivenite (Cu₂AsO₄OH): Olive-green copper arsenate.
Libethenite (Cu₂PO₄OH): Dark green copper phosphate.
Cornetite (Cu₃PO₄(OH)₃): Blue copper phosphate.
Pseudomalachite (Cu₅(PO₄)₂(OH)₄): Dark green copper phosphate.
Langite (Cu₄SO₄(OH)₆·2H₂O): Blue-green copper sulfate.
Linarite (PbCu(SO₄)(OH)₂): Deep blue lead copper sulfate.
Caledonite (Cu₂Pb₅(SO₄)₃CO₃(OH)₆): Blue-green lead copper sulfate carbonate.
Aurichalcite ((Zn,Cu)₅(CO₃)₂(OH)₆): Pale green to blue zinc copper carbonate.
Copper Compounds and Alloys
Industrial Copper Compounds:
- Copper(I) oxide (Cu₂O): Antifouling paints, fungicides
- Copper(II) oxide (CuO): Pigments, catalysts
- Copper sulfate (CuSO₄): Agriculture, algicides
- Copper chloride (CuCl₂): Catalysts, wood preservatives
- Copper nitrate (Cu(NO₃)₂): Catalysts, colorants
- Copper carbonate (CuCO₃): Pigments, fungicides
- Copper acetate (Cu(C₂H₃O₂)₂): Pesticides, pigments
- Copper hydroxide (Cu(OH)₂): Fungicides, pigments
- Bordeaux mixture (CuSO₄ + Ca(OH)₂): Agricultural fungicide
- Cuprous cyanide (CuCN): Electroplating
- Copper nanoparticles: Antimicrobial applications
Important Copper Alloys:
- Bronze (Cu-Sn): Historical and modern engineering applications
- Brass (Cu-Zn): Musical instruments, fixtures
- Cupronickel (Cu-Ni): Coins, marine applications
- Aluminum bronze (Cu-Al): Marine and industrial applications
- Beryllium copper (Cu-Be): Spring materials, tools
- Phosphor bronze (Cu-Sn-P): Electrical contacts
- German silver (Cu-Ni-Zn): Decorative applications
- Monel (Cu-Ni): Corrosion-resistant alloy
Total copper-derived minerals and compounds: Approximately 200+ natural minerals and 50+ synthetic compounds and alloys
6. Manganese: The Essential Metallurgical Element
Overview and Properties
Manganese (Mn, atomic number 25) is the third most abundant transition metal in Earth’s crust after iron and titanium. Despite being less familiar to the general public, manganese is absolutely critical for steel production, making it one of the most important industrial metals. Approximately 90% of all manganese is used in metallurgy, primarily for steel alloying and deoxidizing.
Primary Manganese Oxide Minerals
Pyrolusite (MnO₂): The most important manganese ore, containing 63.2% manganese. This black to steel-gray mineral is the most stable manganese oxide under surface conditions.
Manganite (MnO(OH)): Dark gray to black manganese hydroxide oxide containing 62.4% manganese, commonly forming prismatic crystals.
Hausmannite (Mn₃O₄): Brownish-black manganese oxide with 72% manganese content, forming tetragonal crystals.
Braunite (Mn₇SiO₁₂): Complex manganese silicate oxide containing about 60% manganese.
Bixbyite ((Mn,Fe)₂O₃): Black manganese iron oxide, cubic crystal system.
Psilomelane (BaMn₉O₁₆(OH)₄): Barium manganese oxide, actually a mix of several minerals including romanechite.
Romanechite (BaMn₉O₁₆(OH)₄): The proper name for what was called psilomelane, containing 45-60% manganese.
Cryptomelane (KMn₈O₁₆): Potassium manganese oxide, a common component of manganese nodules.
Hollandite (BaMn₈O₁₆): Barium manganese oxide similar to cryptomelane.
Todorokite ((Na,Ca,K,Ba,Sr)₁₋ₓ(Mn,Mg,Al)₆O₁₂·3-4H₂O): Hydrous manganese oxide found in marine nodules.
Birnessite ((Na,Ca)₀.₅(Mn⁴⁺,Mn³⁺)₂O₄·1.5H₂O): Hydrous manganese oxide, important in ocean floor nodules.
Nsutite (γ-MnO₂): A polymorph of pyrolusite with defect structure.
Ramsdellite (MnO₂): Another polymorph of pyrolusite with different crystal structure.
Manganese Carbonate and Silicate Minerals
Rhodochrosite (MnCO₃): Pink to red manganese carbonate containing 47.8% manganese, prized as a gemstone and ore mineral.
Rhodonite (MnSiO₃): Pink to red manganese silicate with about 42% manganese content, popular ornamental stone.
Spessartine (Mn₃Al₂(SiO₄)₃): Orange to red manganese aluminum garnet, gem-quality.
Tephroite (Mn₂SiO₄): Gray-green to olive-green manganese olivine.
Bustamite ((Mn,Ca)₃Si₃O₉): Pink to brownish manganese calcium silicate.
Pyroxmangite (MnSiO₃): Pink to reddish-brown manganese silicate, similar to rhodonite.
Glaucochroite (CaMnSiO₄): Calcium manganese silicate.
Sonolite (Mn₉(SiO₄)₄(OH,F)₂): Complex manganese silicate hydroxide.
Manganese Phosphate and Other Minerals
Triplite ((Mn,Fe)₂PO₄F): Brown to black manganese iron phosphate fluoride.
Lithiophilite (LiMnPO₄): Lithium manganese phosphate, important in battery research.
Hureaulite (Mn₅(PO₃OH)₂(PO₄)₂·4H₂O): Hydrous manganese phosphate.
Fairfieldite (Ca₂Mn(PO₄)₂·2H₂O): Calcium manganese phosphate.
Hetaerolite (ZnMn₂O₄): Zinc manganese oxide.
Jacobsite (MnFe₂O₄): Manganese iron oxide spinel.
Franklinite ((Fe,Mn,Zn)(Fe,Mn)₂O₄): Complex zinc manganese iron oxide.
Alabandite (MnS): Manganese sulfide, iron-black color.
Manganosite (MnO): Green manganese oxide, rare.
Neptunite (KNa₂Li(Fe,Mn)₂Ti₂Si₈O₂₄): Complex sodium potassium lithium manganese iron titanium silicate.
Manganese-bearing Mixed Minerals
Spinel Group: Various manganese-bearing spinels Epidote Group: Piemontite (manganese epidote) Amphibole Group: Tirodite (manganese amphibole) Tourmaline Group: Various manganese-bearing tourmalines Zeolite Group: Several manganese-exchanged zeolites
Manganese Compounds
Manganese Oxides and Hydroxides:
- Manganese dioxide (MnO₂): Batteries, oxidizing agent
- Manganese(II) oxide (MnO): Chemical synthesis
- Manganese(III) oxide (Mn₂O₃): Pigments, catalysts
- Manganese(II,III) oxide (Mn₃O₄): Ferrites, pigments
- Manganese(VII) oxide (Mn₂O₇): Powerful oxidizing agent
- Manganese hydroxide (Mn(OH)₂): Precursor compound
Manganese Salts:
- Manganese sulfate (MnSO₄): Fertilizers, animal feed
- Manganese chloride (MnCl₂): Chemical synthesis
- Manganese carbonate (MnCO₃): Dietary supplements
- Manganese nitrate (Mn(NO₃)₂): Ceramics, catalysts
- Manganese acetate (Mn(C₂H₃O₂)₂): Textile dyeing
- Manganese phosphate (Mn₃(PO₄)₂): Corrosion protection coatings
Permanganates:
- Potassium permanganate (KMnO₄): Water treatment, oxidizing agent
- Sodium permanganate (NaMnO₄): Industrial oxidizer
Manganese Alloys:
- Ferromanganese: 70-80% Mn, steelmaking
- Silicomanganese: 60-70% Mn, 15-30% Si, steelmaking
- Manganin (Cu-Mn-Ni): Electrical resistor alloys
- Manganese bronze: Various copper-manganese alloys
Advanced Manganese Compounds:
- Lithium manganese oxide (LiMn₂O₄): Battery cathode material
- Manganese dioxide nanostructures: Energy storage
- Manganese ferrites (MnFe₂O₄): Magnetic materials
- Manganese complexes: Various coordination compounds for catalysis
Total manganese-derived minerals and compounds: Approximately 80-100 natural minerals and 40+ synthetic compounds
Comparative Analysis: Mineral Diversity
Summary of Mineral Counts
Based on the comprehensive review above, here’s a comparison of mineral diversity for each element:
- Copper: 200+ natural minerals, 50+ synthetic compounds and alloys
- Copper forms the most diverse range of minerals due to its multiple oxidation states and ability to combine with numerous anions
- Silver: 200+ natural minerals, 30+ synthetic compounds
- Silver’s extensive mineral diversity stems from its occurrence in various sulfides, sulfosalts, and halides
- Manganese: 80-100 natural minerals, 40+ synthetic compounds
- Manganese’s variable oxidation states enable formation of diverse oxide, carbonate, and silicate minerals
- Platinum: 25-30 minerals (including PGMs), numerous alloys
- Platinum group minerals occur together, expanding the family of related minerals
- Gold: 30-40 natural minerals, 20+ synthetic compounds
- Gold’s chemical inertness limits mineral diversity, with most occurring as native gold
- Lithium: 15-20 major minerals, 20+ compounds
- Lithium’s limited mineral diversity reflects its relatively simple chemistry, though it forms important battery compounds
Industrial Significance and Applications
Lithium Applications
- Rechargeable batteries (electric vehicles, smartphones, power tools)
- Ceramics and glass (low thermal expansion products)
- Lubricating greases
- Pharmaceutical industry (mood stabilization)
- Air purification (CO₂ absorption)
- Nuclear industry (tritium production)
- Aluminum alloys
Platinum Applications
- Catalytic converters (automotive emissions control)
- Petroleum refining catalysts
- Chemical manufacturing catalysts
- Jewelry (prestige and durability)
- Dental and medical devices
- Electronics (hard disk drives, thermocouples)
- Glass manufacturing equipment
- Fuel cells (hydrogen economy)
- Cancer treatment drugs (cisplatin)
Silver Applications
- Photography (historical, declining)
- Electrical contacts and conductors
- Solar panels (photovoltaic cells)
- Medical devices and antimicrobial coatings
- Water purification
- Mirrors and reflective coatings
- Jewelry and silverware
- Electronics (switches, keyboards)
- Brazing and soldering
- Catalysts in chemical production
Gold Applications
- Jewelry and decorative arts (50% of demand)
- Investment and monetary reserve
- Electronics (connectors, bonding wire)
- Dentistry (fillings, crowns, bridges)
- Aerospace (satellite components, reflective coatings)
- Medical devices and treatments
- Glassmaking (ruby glass coloring)
- Nanotechnology (drug delivery, diagnostics)
Copper Applications
- Electrical wiring and motors (60% of usage)
- Plumbing and water supply systems
- Building construction (roofing, HVAC)
- Electronics (printed circuit boards)
- Transportation equipment
- Industrial machinery
- Telecommunications
- Renewable energy systems (wind turbines, solar)
- Antimicrobial surfaces
- Alloying element in numerous metals
Manganese Applications
- Steel production (deoxidizer and alloying, 90% of use)
- Aluminum alloys (beverage cans)
- Batteries (alkaline and lithium-ion)
- Animal feed supplements
- Fertilizers
- Pigments and colorants in glass and ceramics
- Water treatment (removal of iron and sulfur)
- Chemical manufacturing
- Welding rod coatings
- Fuel additives (historical)
Extraction and Processing
Common Extraction Methods
Mining Techniques:
- Open-pit mining (large, near-surface deposits)
- Underground mining (deep, high-grade deposits)
- Placer mining (gold, platinum in alluvial deposits)
- Solution mining/brine extraction (lithium from salt lakes)
- Deep-sea mining (manganese nodules, future potential)
Processing Methods:
- Pyrometallurgy: High-temperature smelting and refining (copper, gold)
- Hydrometallurgy: Chemical leaching and solution processing (lithium, copper, gold, silver)
- Electrowinning: Electrochemical extraction from solutions (copper, manganese)
- Flotation: Concentration of sulfide minerals (copper, silver, platinum)
- Gravity separation: Concentration of heavy minerals (gold, platinum)
- Cyanidation: Gold and silver extraction using cyanide solutions
- Evaporation: Lithium extraction from brines
- Roasting: Converting sulfides to oxides (copper, manganese)
Environmental and Economic Considerations
Environmental Impacts
All mining and processing of these minerals carry environmental consequences:
- Habitat destruction and biodiversity loss from mining operations
- Water consumption and contamination from processing chemicals
- Air pollution from smelting operations
- Soil degradation and erosion from open-pit mining
- Energy consumption and associated carbon emissions
- Waste generation including tailings and slag
- Heavy metal contamination in surrounding ecosystems
Sustainability Initiatives
Modern mining increasingly focuses on sustainability:
- Recycling programs: Particularly effective for gold, silver, copper, and platinum
- Improved extraction efficiency: Reducing waste and environmental footprint
- Renewable energy use: Powering mining operations with clean energy
- Responsible sourcing: Certification programs and ethical mining standards
- Remediation technologies: Restoring mined lands and treating contaminated water
- Urban mining: Recovering metals from electronic waste
- Circular economy approaches: Designing products for metal recovery
Economic Importance
These six metals represent significant portions of global economic activity:
- Lithium: Market valued at $6-8 billion annually, rapidly growing with EV adoption
- Platinum: Annual production of ~180-200 tonnes, market value ~$8-10 billion
- Silver: Annual production of ~25,000 tonnes, market value ~$20-25 billion
- Gold: Annual production of ~3,000 tonnes, market value ~$150-200 billion
- Copper: Annual production of ~20-25 million tonnes, market value ~$150-200 billion
- Manganese: Annual production of ~19-20 million tonnes of ore, value ~$10-15 billion
Future Outlook and Emerging Technologies
Growing Demand Sectors
Energy Transition:
- Lithium-ion batteries for electric vehicles and grid storage
- Silver in solar photovoltaic systems
- Copper in renewable energy infrastructure
- Platinum in hydrogen fuel cells
- Manganese in advanced battery chemistries
Electronics Miniaturization:
- Gold and silver in high-performance connectors
- Copper in semiconductor interconnects
- Platinum in hard drive components
Medical Advances:
- Silver nanoparticles for antimicrobial applications
- Gold nanoparticles for targeted drug delivery
- Platinum compounds in cancer chemotherapy
- Copper in antimicrobial surfaces
Catalysis and Chemical Industry:
- Platinum group metals in emissions control
- Manganese compounds in water treatment
- Copper catalysts in organic synthesis
Supply Chain Challenges
- Geographic concentration: Major deposits limited to specific regions
- Geopolitical risks: Mining concentrated in politically unstable areas
- Resource depletion: High-grade deposits becoming scarcer
- Processing bottlenecks: Limited refining capacity for some metals
- Environmental regulations: Increasing restrictions on mining activities
Technological Innovations
Extraction Technologies:
- In-situ leaching for reduced surface disturbance
- Bioleaching using bacteria to extract metals
- Direct lithium extraction (DLE) from brines
- Deep-sea mining technologies for manganese nodules
- Selective extraction methods for complex ores
Material Science Advances:
- Lithium-sulfur and lithium-air batteries
- Platinum-free catalysts for fuel cells
- Graphene-enhanced copper conductors
- High-entropy alloys incorporating multiple metals
- Nanostructured materials with enhanced properties
Recycling Technologies:
- Improved battery recycling for lithium recovery
- Electronic waste processing for precious metals
- Closed-loop manufacturing systems
- Urban mining initiatives
- Automated sorting and separation technologies
Conclusion
Lithium, platinum, silver, gold, copper, and manganese represent six of the most important chemical minerals supporting modern civilization. From the estimated 15-20 lithium minerals to the remarkable 200+ copper minerals, these elements form hundreds of distinct mineral species and countless synthetic compounds that enable technologies ranging from ancient crafts to cutting-edge batteries and catalysts.
The diversity of these minerals reflects the complexity of Earth’s geochemical processes operating over billions of years. Each element’s unique chemistry determines both its mineral diversity and its technological applications. Copper’s versatility in forming diverse compounds makes it irreplaceable in electrical applications. Silver’s antimicrobial properties and conductivity create unique opportunities in medicine and electronics. Gold’s inertness and beauty ensure its continued role as a store of value. Platinum’s catalytic properties enable cleaner transportation. Lithium’s electrochemical characteristics power the energy storage revolution. Manganese’s role in steel production underpins modern infrastructure.
As humanity faces the dual challenges of resource scarcity and environmental sustainability, understanding these minerals becomes increasingly critical. The transition to renewable energy, electric transportation, and circular economies will depend heavily on our ability to extract, process, utilize, and recycle these essential elements responsibly. The future will likely see continued innovation in extraction technologies, development of substitutes and alternatives, and increasingly sophisticated recycling systems to ensure these valuable resources remain available for generations to come.
The story of these six chemical minerals is ultimately the story of human technological development—from the first copper tools to modern lithium batteries, from ancient gold coins to platinum cancer drugs. As we continue to discover new applications and develop more sustainable practices, these minerals will remain fundamental to human progress and prosperity.




Be First to Comment